Background Information

| Breed Card | |
| Breed | Cemani chicken |
| Species taxonomy | Gallus gallus domesticus |
| Classification | Traditional |
| Region | Indonesia |
| Purpose | Fancy |
The Ayam Cemani ("ayam" means chicken in Indonesian and Malay) is a very unusual and exotic breed from Indonesia. Cemani can either mean the "village of Cemani" in local dialect or "solid black" in Sanskrit. It possibly originated in Sumatra where the original breed is now extinct. People of wealth and community standing have these birds as status symbols and take great care of them. They are thought of as good luck charms, with the blood and other parts of the bird being used in traditional medicine preparations. The Ayam Cemani is said to have magical powers and can facilitate communication between the living and the spirit world. As such, it is used as a sacrificial bird to please the gods, its' flesh is rarely eaten in Indonesia. They were first imported to Europe in 1998 by a Dutchman named Jan Steverink.
Cemani chickens have a dominant phenotype of hyperpigmentation showing mostly black, including feathers, beak, and internal organs. The roosters weigh in around 4.5-6.5 lb, with the hens coming in at 3.5-4.5 lb, respectively. In appearance they are strong and muscular with close fitting feathers, not unlike a game bird. On average they will lay around 80 eggs per year which is around 1 egg per week. In comparison to the size of the hen the eggs are quite large and they are cream colored with a very slight pink tint (not black).
Variants Annotation&Density
| Annotation | Population SNP | Total SNP | Percentage SNP | Population INDEL | Total INDEL | Percentage INDEL |
|---|---|---|---|---|---|---|
| downstream | 31570 | 650455 | 4.8535% | 2738 | 104154 | 2.6288% |
| exonic;splicing | 3 | 188 | 1.5957% | 0 | 0 | 0% |
| exonic_unknown | 15 | 582 | 2.5773% | 4 | 82 | 4.878% |
| frameshift_deletion | 0 | 0 | 0% | 87 | 15977 | 0.5445% |
| frameshift_insertion | 0 | 0 | 0% | 104 | 13308 | 0.7815% |
| intergenic | 745320 | 15129055 | 4.9264% | 59845 | 2238383 | 2.6736% |
| intronic | 955515 | 17735594 | 5.3876% | 81674 | 2641780 | 3.0916% |
| ncRNA_exonic | 19968 | 400185 | 4.9897% | 1590 | 54342 | 2.9259% |
| ncRNA_exonic;splicing | 14 | 231 | 6.0606% | 0 | 43 | 0% |
| ncRNA_intronic | 200545 | 3728327 | 5.379% | 17271 | 575920 | 2.9989% |
| ncRNA_splicing | 124 | 2341 | 5.2969% | 18 | 478 | 3.7657% |
| ncRNA_UTR5 | 0 | 0 | 0% | 0 | 18 | 0% |
| nonframeshift_deletion | 0 | 0 | 0% | 92 | 8777 | 1.0482% |
| nonframeshift_insertion | 0 | 0 | 0% | 20 | 4784 | 0.4181% |
| nonsynonymous | 7379 | 336233 | 2.1946% | 0 | 0 | 0% |
| splice_acceptor | 18 | 750 | 2.4% | 16 | 964 | 1.6598% |
| splice_donor | 22 | 1076 | 2.0446% | 9 | 763 | 1.1796% |
| splice_donor_acceptor | 0 | 0 | 0% | 2 | 45 | 4.4444% |
| splice_UTR5 | 9 | 400 | 2.25% | 0 | 106 | 0% |
| splie_Others | 0 | 0 | 0% | 8 | 654 | 1.2232% |
| startloss | 23 | 671 | 3.4277% | 1 | 51 | 1.9608% |
| stopgain | 85 | 4175 | 2.0359% | 7 | 1252 | 0.5591% |
| stoploss | 13 | 353 | 3.6827% | 0 | 63 | 0% |
| synonymous | 17518 | 548813 | 3.192% | 0 | 0 | 0% |
| upstream | 28946 | 679592 | 4.2593% | 2166 | 98760 | 2.1932% |
| upstream;downstream | 2167 | 57451 | 3.7719% | 197 | 9367 | 2.1031% |
| UTR3 | 16103 | 361040 | 4.4602% | 1575 | 61415 | 2.5645% |
| UTR5 | 3506 | 112990 | 3.1029% | 231 | 16935 | 1.364% |
| UTR5;UTR3 | 95 | 2524 | 3.7639% | 8 | 381 | 2.0997% |
| Total | 2028958 | 39753026 | 5.1039% | 167663 | 5848802 | 2.8666% |
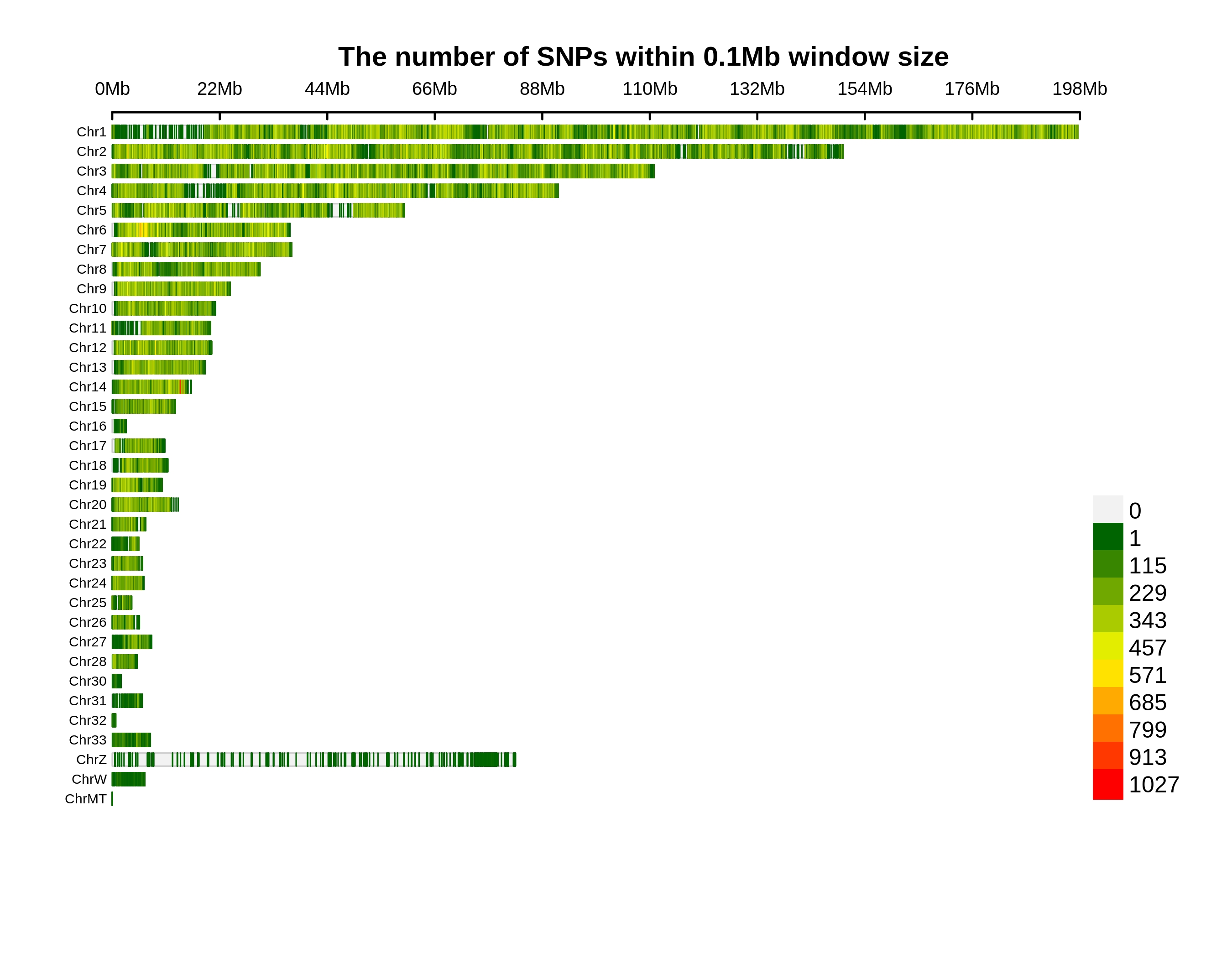

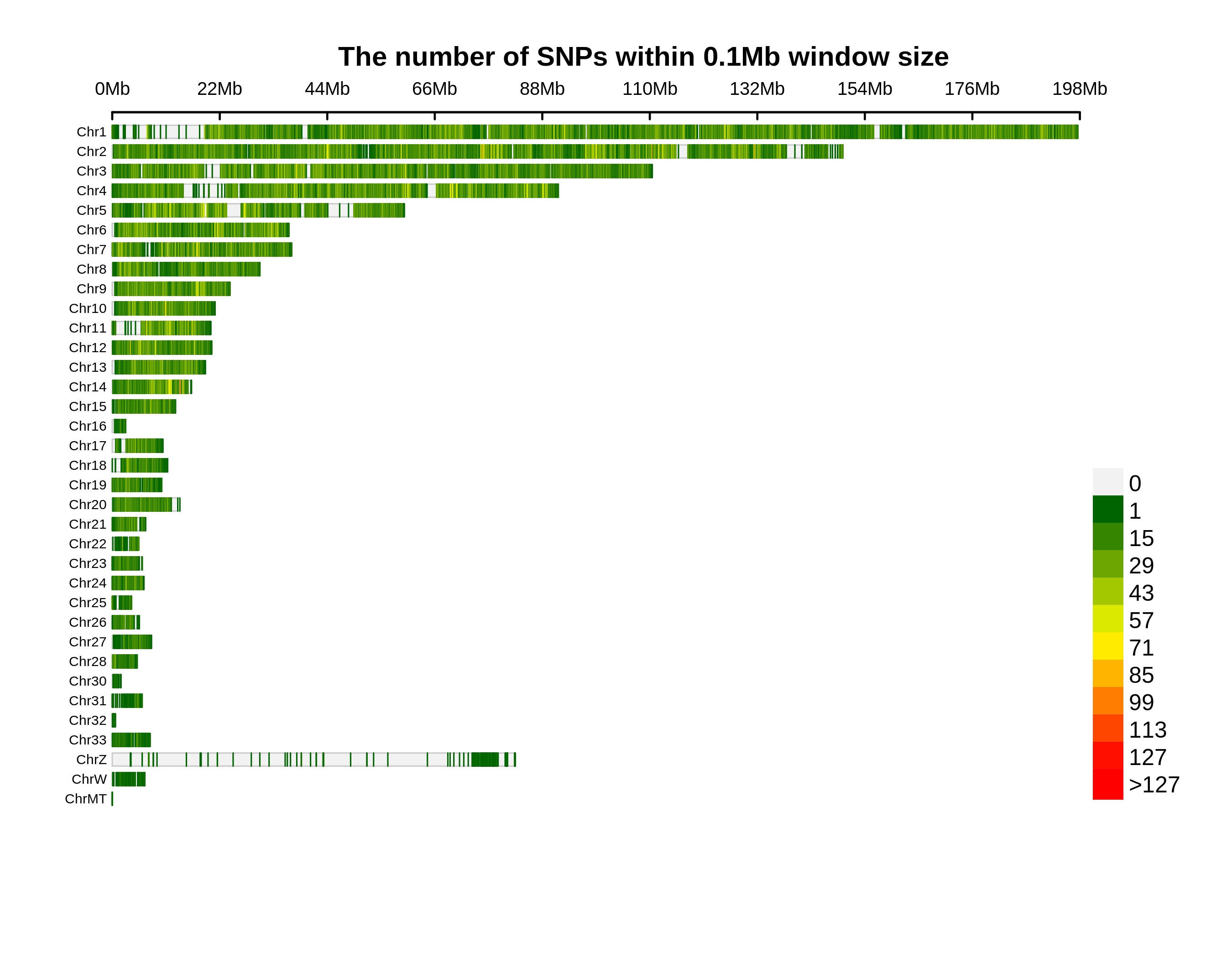

Genetic Differentiation
Summary
Genetic affinities of target population in the context of worldwide populations are measured by pairwise FST between target population and references. Smaller FST value indicates closer relationship. Regions represented by different colors are indicated above.
Genetic Affinity
Summary
Genetic affiliation and population structure are shown by PCA plots. After removing G. g. bankiva, G. g. jabouillei, and some G. g. gallus individuals as outliers to other Red Jungle Fowl, the dataset contains 1,915 samples from domestic chicken and Red Jungle Fowl. User can add any populations with interests to show with the target population under this PCA context by using the item of “Add”.
ADMIXTURE Analysis
Summary
The inference of populations and individual ancestries is revealed by ADMIXTURE clustering. Length of each colored bar represents the proportion of proposed ancestry in the sample. User can add any populations with interests to compare with the target population by using the item of “Add”. The number of proposed ancestries is determined by “which K”.
Runs of Homozygosity
Summary
Runs of homozygosity (ROH) indicates long tracts of homozygous genotypes inherited from identical haplotypes of a common ancestor. Larger populations have fewer, shorter ROH, whereas isolated or bottlenecked populations have more, somewhat longer ROH. Admixture brings the fewest ROH, whereas inbreeding causes long ROH. The level of ROH is measured by number and length. The length of ROH can be defined in “ROH range”. User can add any populations with interests to compare with the target population by using the item of “Add”.
Linkage Disequilibrium Decay
Summary
Linkage disequilibrium (LD) decay is characterized by squared correlations (r²) of all SNPs frequencies against the physical distances between SNPs. User can add any populations with interests to compare with the target population by using the item of “Add”.
Demographic History
Summary
The changes of effective population size through time is inferred by SMC++. User can add any populations with interests to compare with the target population by using the item of “Add”.
Selection
Summary
Selective signals of the target population are detected by different methods. X axis indicates physical position of specific chromosomal region with interests which can be defined by “Region”. The gene annotation is shown below. Y axis of left (YL) indicates the values of Pi-ratio of -log2(πRJF/πTarget) or composite likelihood ratio (CLR) of SweeD. The levels of statistical significance are noted with different colors. Y axis of right (YR) shows the values of Fst (Target vs. Red Jungle Fowl), Pi, or Tajima’s D with blue line with sliding window approach. The genomic window size and step size for Tajima’s D are 5 kb and 5 kb, respectively. The genomic window size and step size for Fst and Pi are 10 kb and 5 kb, respectively. The methods are defined by “Method-YL” and “Method-YR”.